Funds Raised
Citizen Scientist Engaged
The Recreational Fisheries Research Institute, Inc. (RFRI)
The RFRI has long been an integral part in the development of improved fishery data collection, fish habitat restoration,and the fostering of a catch and release, citizen-scientist driven movement amongst Louisiana’s recreational and tournament anglers. The partnership of recreational anglers and scientist help everyone conserve the resource while maintaining access to great times spent outdoors and on the water. A variety of projects have branched out from this common goal and each one has been successful in its focused goals. Learn more about our mission.
The RFRI has long been an integral part in the development of improved fishery data collection, fish habitat restoration, and the fostering of a catch and release, citizen-scientist driven movement amongst Louisiana’s recreational and tournament anglers. The partnership of recreational anglers and scientist help everyone conserve the resource while maintaining access to great times spent outdoors and on the water. A variety of projects have branched out from this common goal and each one has been successful in its focused goals. Learn more about our mission.
The RFRI has long been an integral part in the development of improved fishery data collection, fish habitat restoration, and the fostering of a catch and release, citizen-scientist driven movement amongst Louisiana’s recreational and tournament anglers. The partnership of recreational anglers and scientist help everyone conserve the resource while maintaining access to great times spent outdoors and on the water. A variety of projects have branched out from this common goal and each one has been successful in its focused goals. Learn more about our mission.
The RFRI has long been an integral part in the development of improved fishery data collection, fish habitat restoration, and the fostering of a catch and release, citizen-scientist driven movement amongst Louisiana’s recreational and tournament anglers. The partnership of recreational anglers and scientist help everyone conserve the resource while maintaining access to great times spent outdoors and on the water. A variety of projects have branched out from this common goal and each one has been successful in its focused goals. Learn more about our mission.
In a time when technology often holds us captive, RFRI understands the importance of getting outside, being physically active, embracing teamwork, and connecting with nature and each other.
The creation of inshore reefs provides two main benefits: they assist in reducing coastal erosion and restore hard bottom and essential fish habitats where it had previously existed.
In Texas and Florida, supplemental, hatchery-based stocking, combined with standard management practices, has resulted in a successful and sustaining program of Red Drum enhancement.
With low entry fees, tournaments allow anglers to fish close to home and compete for cash prizes while simultaneously giving back to the resource through tag and release fishing.
Fishing tournaments continue to grow in popularity and diversity of formats, but putting them on has never been easier with the development of the RFRI Leaderboard software.
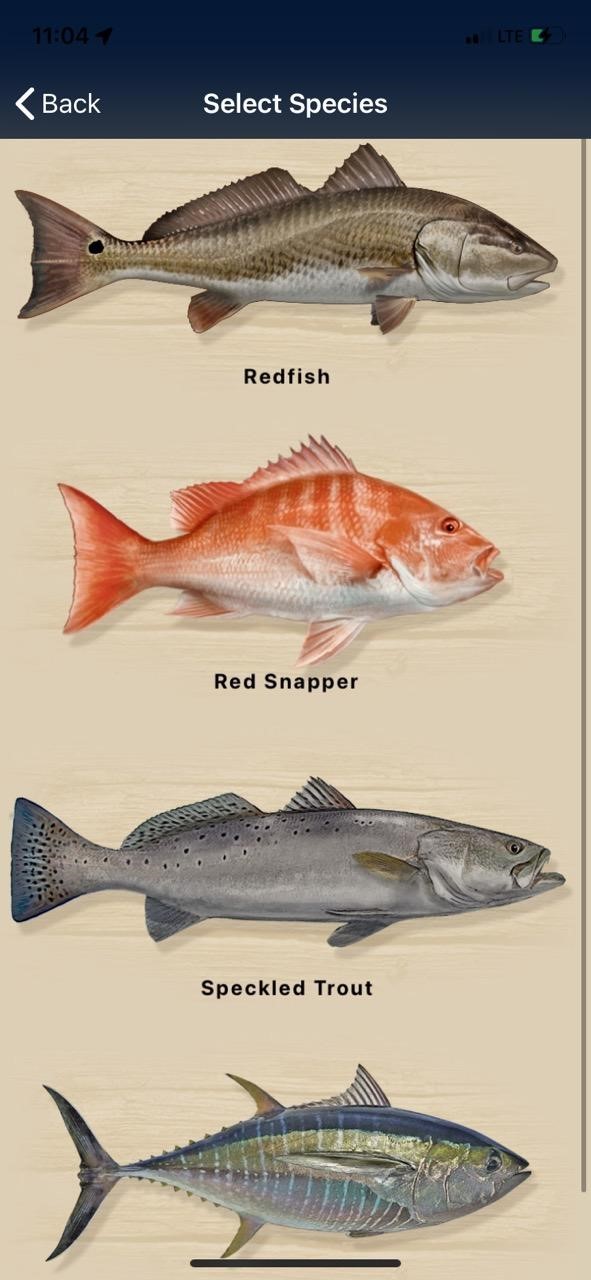
RFRI has partnered with the Wye Foundation on the Fishial.AI Fish ID Project with the goal of making highly accurate fish identification “Fishial Recognition” possible.
Fishing tournaments continue to grow in popularity and diversity of formats, but putting them on has never been easier with the development of the RFRI Leaderboard software.
The RFRI has long been an integral part in the development of improved fishery data collection, fish habitat restoration, and the fostering of a catch and release, citizen-scientist driven movement amongst Louisiana’s recreational and tournament anglers. The partnership of recreational anglers and scientist help everyone conserve the resource while maintaining access to great times spent outdoors and on the water. A variety of projects have branched out from this common goal and each one has been successful in its focused goals. Learn more about our mission.
Our Mission
RFRI is a non-profit organization formed exclusively to conduct marine fisheries restoration through education and public involvement. Fostering diverse partnerships and engaging anglers in data collection are key objects leading to successful projects. For the past 20 years RFRI has built a team of citizen scientists to gather data which could be used proactively rather than the industry standard, reactive.
This is accomplished by establishing community-based fund-raising events where marine conservation and fish habitat restoration and protection are at the focal point. All of these events are organized and executed by volunteers. Experts are invited to present information on the conservation front, educating anglers on actions they can take to be part of the restoration effort.
Marine Fisheries Restoration Through Education & Public Involvement.
Download Anglers Almanac
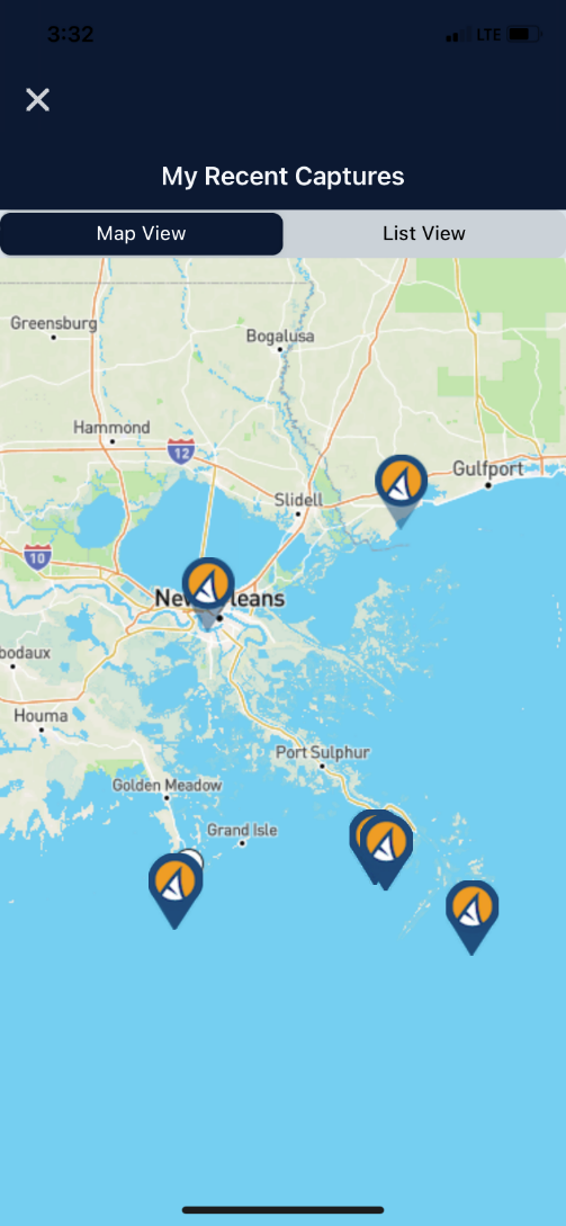
Turn your phone into one of your favorite tools while also turning your catch into citizen scientist data that can be utilized to modernize fish management.

Events

Derby Day
Sat, April 13, 2024 5:30 - till
Desi Vega's Steakhouse
1950 N Highway 190 Covington, LA 70433
Annual RFRI Gala! Join us for a day at the Races, Derby Style! Call 504-415-7186 or email RFRI@charter.net or Click Here

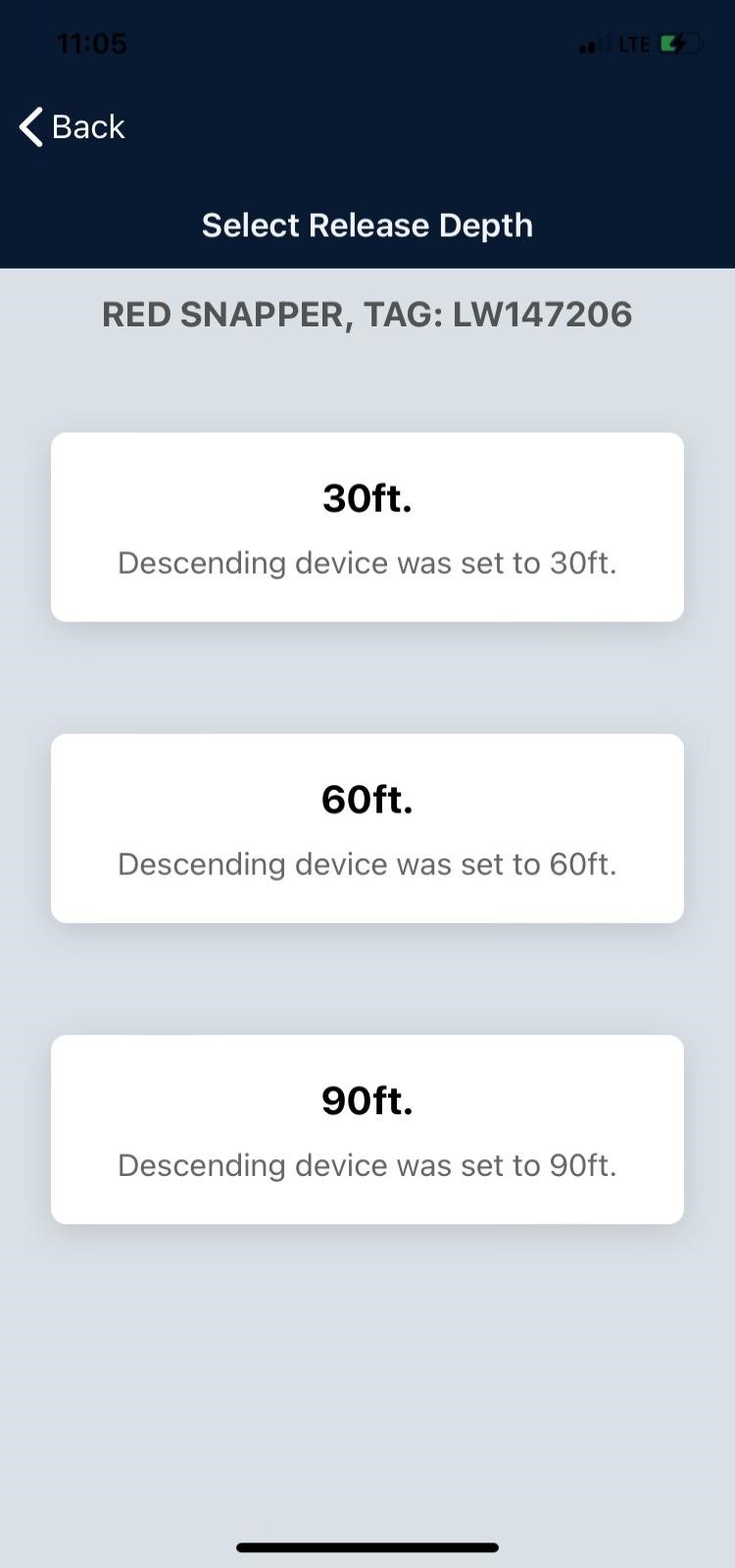
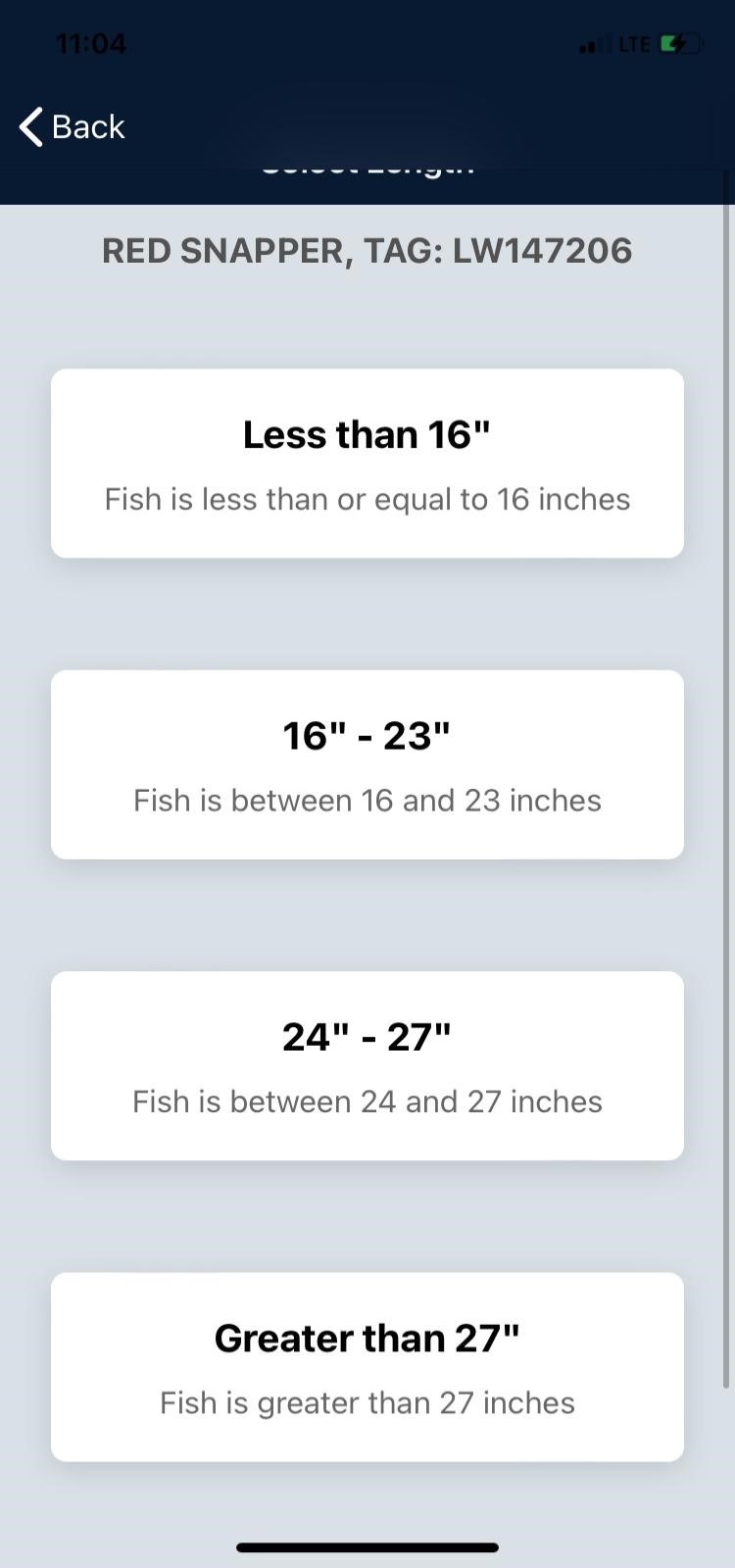
TAG IT Red Snapper Tourney
The 2024 Red Snapper season will begin on Monday, April 15, 2024, season will run 7 days a week with a 4 fish limit & 16-inch minimum length.
Gulf Of Mexico
Call 504-415-7186 or email RFRI@charter.net or Click Here
Recent News

02/18/2024
RFRI Habitat Enhancement Program Deploys Christmas Trees & Recycled Glass at Fountaineblue State Park

01/10/2024
RFRI TAG IT LA Red Snapper Tournament Receives Grant
test

10/10/2023
RFRI receives Keep Louisiana Beautiful Healthy Communities Grant to make glass recycling more available to St Tammany Residents.

07/22/2023
Bill Shedd Owner of AFTCO Receives Conservation Award from RFRI

07/03/2023
RFRI receives 1st ever redfish stocking permit in Louisiana!

03/23/2023
RFRI Habitat Enhancement Christmas Trees Fountaineblue State Park

09/03/2022
2022 TAG IT Louisiana Tournament Youth Winner

08/02/2022
RFRI tags 100 fish at the Ride the Bull Kayak Tournament
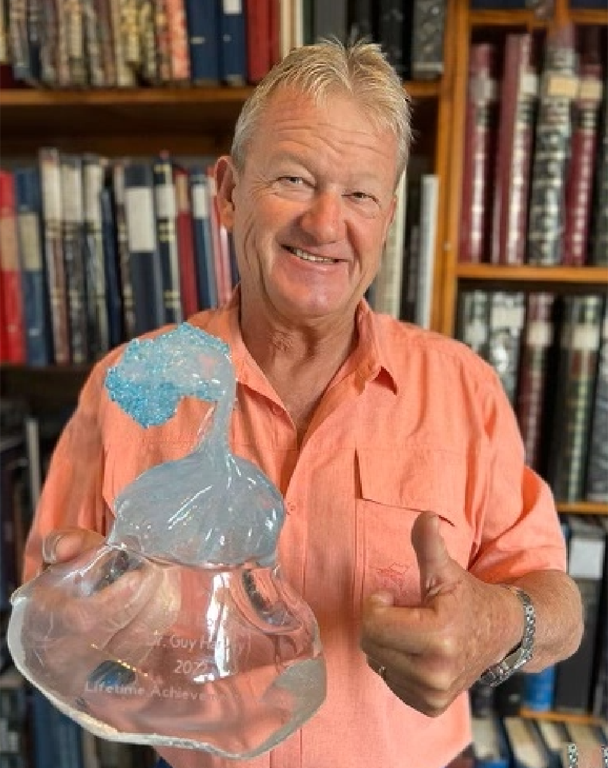
07/04/2022
Guy Harvey Receives Conservation Award from RFRI

12/01/2020
RFRI & Brother Martin Beach Clean Up sponsored by Chick-fil-A 1-90
ALL DONATIONS ARE TAX DEDUCTIBLE.
Managing the future of fishing in Louisiana is a monumental task and in order for the RFRI to tackle fisheries, habitat, and coastal restoration projects, funding is required. Your contributions will greatly protect, enhance and maintain the Gulf Coast Marine Fishery through science, education and public involvement.
Our Sponsors
Family owned and operated, the American Fishing Tackle Company (AFTCO) represents unparalleled quality, performance, and reliability when it counts most.
YETI is a global designer, retailer, and distributor of innovative outdoor products.
Established in 1921, Shimano today globally operates three key businesses, with sales offices and factories all over the world. We will continue supplying “captivating products” to help promote the bicycle and sports fishing cultures.

Born on the water in 1983, Costa Sunglasses is celebrating 40 years of making the best sunglasses on the planet and protecting the watery world we call home. For those like us who don’t just spend their life on the water, but fully come to life on it, big days are coming.